Blog Post
Differentiating Chemical Purity Levels: From Reagent Grade to Pharmaceutical Grade
Differentiating Chemical Purity Levels: From Reagent Grade to Pharmaceutical Grade
Navigating the world of chemical purities, one encounters several terms such as reagent grade, purified grade, laboratory grade, ACS grade, and pharmaceutical grade. This article aims to shed light on these distinct purity levels and their applications.
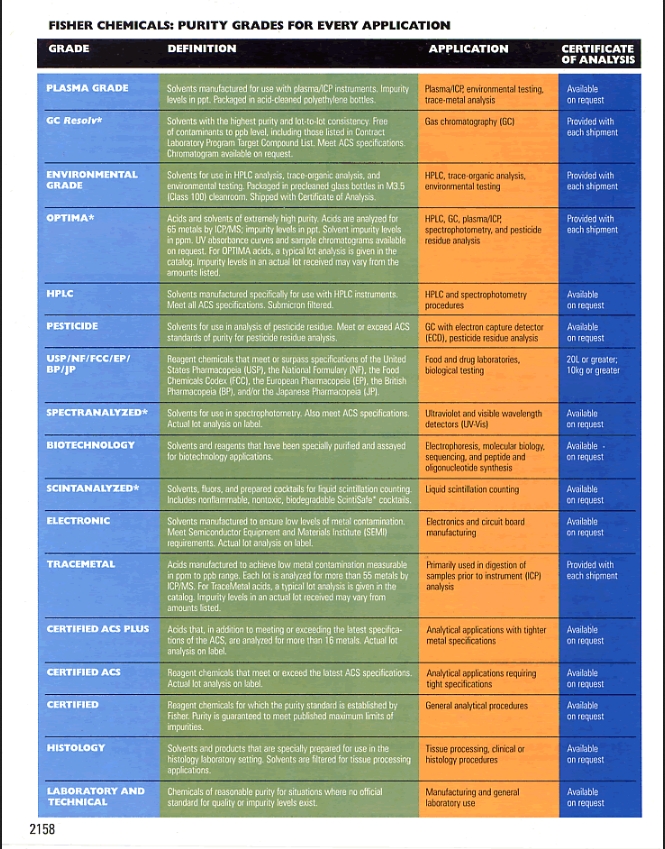
Chemical Purity Grades Defined
Chemical purity signifies how much a substance aligns with its primary intended component. Impurities might arise during production, storage, or even transportation. Below is a simplified breakdown of these grades:
- Reagent Grade:
- Suitable for general laboratory applications.
- Not necessarily pure enough for human consumption.
- Purified Grade:
- Higher purity level than reagent grade but may still contain some impurities.
- Typically used for specific analytical purposes.
- Laboratory Grade:
- Commonly used in educational settings.
- Not as pure as reagent or purified grades but sufficient for teaching and training.
- ACS Grade:
- Meets the purity criteria set by the American Chemical Society (ACS).
- Often used for analytical purposes and critical scientific experiments.
- Pharmaceutical Grade:
- Must adhere to stringent regulatory standards, ensuring it’s safe for human consumption or medical applications.
- Highest purity level, free from harmful contaminants.
Importance of Recognizing the Grades
Safety in Application: Using the correct grade is essential to ensure safety, especially in human-related applications. For instance, pharmaceutical grade chemicals must be devoid of harmful impurities to be fit for medical use.
Cost Implications: Higher purity grades like the pharmaceutical grade often entail more sophisticated purification processes, which can lead to higher costs. Conversely, laboratory grade chemicals, which are suitable for educational purposes, might be more cost-effective.
Regulatory Compliance: Regulatory bodies, such as the FDA, have strict guidelines on chemical purities for pharmaceuticals. Using the correct grade ensures adherence to these standards, facilitating quicker approvals and ensuring public safety.
Conclusion
Being aware of the differences in chemical purity levels, from reagent grade to pharmaceutical grade, is pivotal in ensuring chemicals’ safe and effective use. It’s beneficial for researchers, educators, and healthcare professionals to comprehend these classifications for appropriate and safe application.